
Scheme of Consultation on Research ethics
The office for human research studies helps investigators to get ethical review approval, based on the Ethical Guidelines for Medical and Biological Research Involving Human Subjects.
Consulting on research ethics, provided by the OHRS
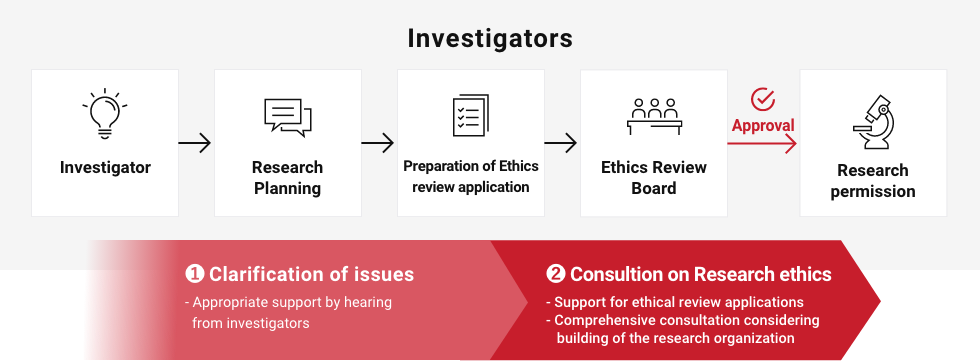
* For the study to get started, the principal investigator(PI) requires not only ethics approval by the independent committee but also premission by the head(s) of institution(s) where PI(s) belong to.
1 – Appropriate support by hearing from investigatorsClarification of issues
Consultation prior to ethical review
Regarding life science / medical research involving human subjects, we will offer some advice about
ethical applications;
– Is ethic examination necessary?
– How to be examined from the viewpoint of research ethics
– Precaution in the ethical viewpoint, in product development
※In this initial stage, we confirm what investigators want to do. ① or ②
| Contents | Support by the office for human research studies | |
|---|---|---|
| ① | Support for ethical review applications (Partial support) |
– Support for preparing materials necessary for making ethical applications, solving ethical issues, etc. – Providing research ethics consultation service in a partial sense |
| ② | Comprehensive consultation including the research organization building (Full support) |
– Comprehensive support related to research ethics and implementation; the building of research organization, operating procedure manuals, in-house rules, etc. necessary for conducting research at the research institution the establishment of education and training system – Providing research ethics consultation services |
Definition of Consultation on Research ethics
It is the advisory service by mutual consent with investigators to protect the health and welfare of research subjects; providing information, clarifying and analyzing ethical issues, and advicing how to implementate, through all stages (research planning, research implementation, consideration, and publication of results).
2 – Support for ethical review applications
– Comprehensive consultation including the research organization buildingConsultation on Research ethics
| Contents | investigators | Support by the office for human research studies | |
|---|---|---|---|
| ① | Support for ethical review applications (Partial support) |
Finalization of research plan Selection of ethics review board |
– Judgement of guidelines conformity. – Guidance to the appropriate committee. |
| Preparation of Ethics review application Research protocol, Building a research organization, and Research protocol, Contract, Opt-in document (written information), Consent form, Consent withdrawal, Opt-out document, etc. |
– Support for preparing materials to apply to the Ethical Review Board, etc. – Guidance on research ethics points to be noted for the research subject and proposal of countermeasures – Solving ethical issues. |
||
| Preparation of presentation materials for Ethical Review Board | – Focused guidance for presentation at the ethics review board. – Guidance on preparing responses to inquiries from the Ethical Review Board. |
||
| ② | Comprehensive consultation including the research organization building (Full support) |
Coordination related to research ethics and research implementation | – Comprehensive support related to research ethics and research implementation; the establishment of systems, in-house manuals, rules, etc. the establishment of education and training systems. |
Contact information
The office for human research studies,
Graduate School of Medicine, Faculty of Medicine,
The university of Tokyo
Zip code 113-0033
7-3-1, Hongo, Bunkyo-ku, Tokyo, Japan
TEL:03-5841-0818(ex:20818)
E-mail:ethics@m.u-tokyo.ac.jp

