Medical Care and Research Ethics

Medical Research is an area which deals with people’s health and lives. Not only is it important to take into account the safety and rights of the participants/subjects in the research, stricter management of personal information is required since the human genome became an important subject of medical research. Social trust in medical research is suffering due to endless counts of research misconduct that is taking place. In other words, the “ethics” of those who engage in medical research is being called into question than ever before.
What then, is ethics in medical research?
Many of you may only have a vague idea of what this is. However, this issue is important not only to those who engage in research, but also to corporations that are involved in research, and to those of the general public.
We will be explaining in clear and simple words the “research ethics” that have recently come under the spotlight.
Medical Care and Research Ethics
Connecting Medical Research and Society
1. What is Ethics?
What comes to your mind when you hear the word “ethics”?
When we look the word up in the Kojien Dictionary, it says it is a word found in the classic Chinese literature “Raiki (book of rules and principles one should follow to maintain social order)” and defined as “Moral rules that govern the relationships among people. Principles that actually set the norms of moral values. Moral value.”
Also, if you look at the 2 letters that comprise the Japanese word for ethics, “RIN-RI”,
RIN means company, people, society
RI means the natural order of things, and reasoning
In other words, RINRI (ethics) can refer to a society’s natural order of things, or simply, “appropriate behavior as human beings.”
Incidentally, the word RINRI was first used in Japan as a translation for the word “ethics” that came in from the West during the Meiji era.
2. What kind of ethics is required in medical care?
There are different kinds of ethics for different kinds of positions and occupations. For example, there is the political ethics for politicians and corporate ethics for corporations. These are the norms that the person engaged in each situation and occupation must follow.
So for those who are involved in medicine, medical ethics is important. This can be broken down into three areas of ethics, as explained below.
a、Research Ethics
These refer to the norms that researchers should follow when engaging in research activity, which basically include adequate explanation to research participants, protection of privacy and human rights and fairness in research activity.
b、Clinical Ethics
These are mainly norms that should be followed in clinical practice. It includes areas such as informed consent and physician’s appropriate judgement and behavior in patients’ blood transfusion, refusal of treatment and end-of-life care.
c、Public Health Ethics
These refer to ethics surrounding public health, such as prevention of epidemics. For example, finding the balance between preventing the spread of avian influenza and restricting social activity.
Among these, “Research Ethics” is the area that requires further understanding.
3. Research Ethics represents the connection to society
This is because “research ethics” is the link between the closed spaces of universities/labs and the general public.
For example, research involving people’s visions was employed in the development of recent VR (virtual reality) games, and likewise of research on the effect of nano-technology on human skin in the development of high-end skincare products. So you see that medical research is involved in the development of general industrial products as well.
Furthermore, it is becoming easier to exchange personal genetic information as genetic testing becomes more widespread.
So as you can see, research ethics is becoming a closer issue to our daily lives than ever before.
Below are some of the serious cases involving research ethics in the past.
Unethical human experimentation conducted during WWII
- Human experimentations conducted by Nazi Germany (such as those using low-pressure chambers and poison gas)
- Human experimentations by the Imperial Japanese Army
Unethical human experimentations conducted after WWII (Japan)
- Human experimentations conducted on infants of the Nagoya City Infant Nursery (1952)
As part of a study of a special form of coliform bacillus, the bacteria were given to infants in the nursery. Some fell critically ill, and some even died. - Human experimentation conducted by Niigata University using Rickettsia tsutsugamushi (1952-1955)
Rickettsia tsutsugamushi were injected to 119 inmates of a psychiatric hospital. Eight of them died and one committed suicide. - The Xenalamine incident (1963)
A pharmaceutical company conducted a placebo-controlled study on 207 of its employees using an unapproved antiviral drug called Xenalamine. Seventeen of the subjects ended up in the hospital, and one died.
Unethical human experimentations conducted after WWII (outside Japan)
- Willowbrook Experiments (1950s)
Mentally disabled children were intentionally given hepatitis to study the spread of the viral infection. - Jewish Chronic Disease Hospital Experiment (1960s)
Elderly patients with dementia were injected with live cancer cells for a study to see the effect a debilitated immune response had on the development of cancer. - Tuskegee Syphilis Study (1934-1972)
An experiment led by a government institution where nearly 400 African-American men of Tuskegee Alabama who suffered syphilis were left untreated. Lumbar punctures were conducted as “treatment”, even after penicillin was discovered as a cure for syphilis.
Actions that violate research ethics can at times put people’s health and life at risk. They can also erode the public’s trust towards research and the researchers themselves.
In fact, research ethics is the connection between society and medical research that should never be severed.
If so, what could be done in order to make sure people abide by research ethics? The clue is in the motion of the pendulum.
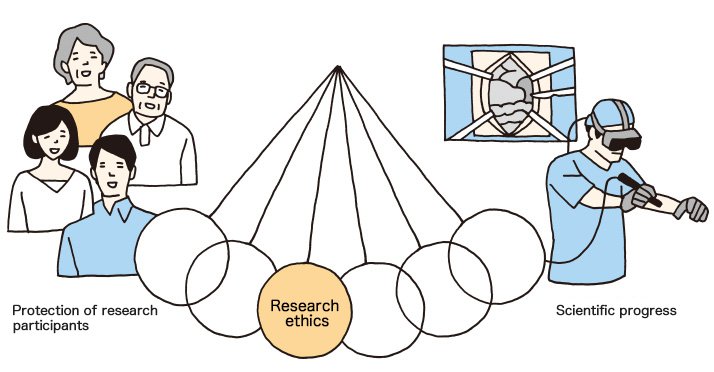
The pendulum of research ethics should not lean towards either side. Sound scientific progress under such circumstances cannot be expected. Still, researchers strive daily to make contributions to science, making them likely to put their research above everything else.
Research participants, on the other hand, lack the technical knowledge, therefore tend to believe their physicians or researchers. What is needed is fair and just supervision by a third party.
4. What is needed: Supervision by a Third Party
A set of International guidelines for research ethics already exists.
Major International Guidelines
- The Nuremberg Code (1947)
It sets 10 ethical principles for human experimentation. As an absolute ethical norm, it states that participation in research must be voluntary. - The Declaration of Helsinki (1964)
Adopted by the WMA (World Medical Association), its principle is that the participant’s welfare must come before the benefit of research. - The Belmont Report (1979)
It set a structured framework of research ethics for the first time, and influenced the ethical guidelines worldwide. - The CIOMS (Council for International Organizations of Medical Sciences) Guidelines (1982)
In cooperation with the WHO, CIOMS arranged the Declaration of Helsinki so that it could be used to guide Third World countries.
The Belmont Report, on which current international guidelines are founded, raises the following 3 principles in research ethics.
Respect for Persons
(Respect should be given to research participants by sharing information such as the purpose of, and risks involved in the research, etc.)
Beneficence
(Research with little social significance or involving too many risks should be avoided, etc.)
Justice
(Research participants should not be selected merely because it is convenient or easy for the researcher, etc.)
You may think what is written above is obvious. But when it comes to assessing the content or individual actions in research that is both complicated and diverse, there is a limit to what one researcher can do. Judging whether or not something is ethical or not is a laborious process for a single researcher, and thus has its limits.
What then becomes important is the role of the “Ethics Committee,” which works as a third-party supervisor.
Each research institution has its own ethics committee which studies and examines whether or not the research conducted in its institution is ethical.
There is also a movement to set up a so-called “Central Ethics Committee” which would play a central role in a unified screening system.
We could expect various advantages if such a system were to be established, since the quality and speed of the examination process would increase, and multiple evaluation of the same research by numerous ethics committees would be avoided in cases of multicenter study undertaken in different facilities.
In the world of medical research today, these ethical guidelines outlined by the ethics committee are considered to be the “rules” in medical research involving human subjects.
5. The need to take interest in Research Ethics
So as we have discussed thus far, the advancement of medical care and research has made the issue of research ethics closer to the general public, allowing situations where without you knowing it, your cells or DNA information could be used in research.
This means that the issue of research ethics is now everybody’s problem.
Therefore in order to protect your own body and personal information, and to guard your research, it is important for both ordinary people and researchers to keep a close watch on the issue of research ethics, and to abide by them.